We study developmental mechanisms of organ formation and growth using the calvaria, the bony skeleton that comprise the top of the skull and encases the brain. Proper formation and growth of the calvaria is critical for the proper accommodation of the growing brain during embryonic and postnatal life. Sustained calvarial bone growth relies on fibrous joints called sutures, which simultaneously act as a barrier between neighboring bones and a source of stem cells to grow bones. In a common congenital abnormality called craniosynostosis, sutures are lost and neighboring bones prematurely fuse, limiting brain growth. In the Farmer lab, we integrate multiple animal models (i.e. mice and zebrafish) with cutting edge genomic, genetic and imaging technologies to decipher the molecular and cellular basis of calvaria development. Our primary questions in the lab seek to under how the skeletogenic and non-skeletogenic mesenchyme is specified, how cranial sutures are established, how calvarial bone and brain growth are coupled, and how disrupting these processes lead to craniofacial defects. These efforts will inform appropriate intervention for the various genetic mutations that cause craniosynostosis.
What are the critical developmental decisions that drive proper calvaria formation?
|
|
The calvaria is derived from neural crest and mesoderm, but the developmental decisions that specify the progenitors that will establish and maintain calvarial bones remain unclear. A longstanding goal of our lab is to decipher the early gene regulatory networks that establish the bone primordia, the developing sutures, and the supporting connective tissues, including meninges. Using novel fate mapping approaches combined with live imaging and genomic technologies, we hope to uncover the molecular mechanisms that facilitate the developmental transitions that lead to a properly formed and maintained cranial suture. |
What are the inter-cellular interactions that drive cranial suture development and stem cell maintenance?
|
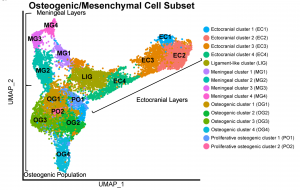
UMAP projection of sc-RNA-seq data from embryonic mouse coronal sutures. |
While the osteogenic subtypes that support calvarial bone growth have been well documented, the identity and function of the non-skeletogenic cell types that reside within and around cranial sutures remain poorly understood. Our recent work uncovered novel non-skeletogenic connective tissues below and above cranial sutures. We are now investigating the role of these tissues during calvaria development, and how these tissues interact with osteogenic subtypes to control bone growth. |
How are biomechanical signals interpreted at cranial sutures to control skeletal stem cells?
|
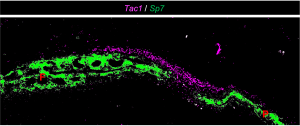
Tac1+ mesenchyme sits above cranial sutures. If and how these cells help calvaria respond to brain growth is under active investigation. |
After sutures are established, calvarial bone growth must be tightly controlled to retain separation between neighboring bones and to accommodate the expanding brain. While external manipulation of cranial sutures has highlighted the capacity of calvarial bones to respond to mechanical load, the natural mechanisms that link brain and bone growth remain poorly understood. We are using genetic and mechanical strategies to determine the basis of brain-bone coupling. |
What is molecular and cellular basis of craniosynostosis?
|
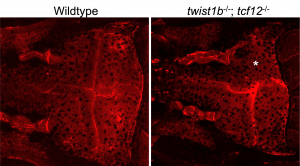
Alizarin Red stain of control and mutant zebrafish skulls. The mutant has unicoronal synostosis (asterisk). |
Craniosynostosis, the premature fusion of neighboring skull bones, has been associated with dozens of genes, but only a small subset have available animal models. Likewise, the precise cellular changes that drive suture loss in patients with craniosynostosis remains poorly evaluated. We aim to create new models of craniosynostosis, informed by clinical observations to functionally associate candidate genes with suture loss as well as to discern the complex regulatory network that is required to establish and maintain cranial sutures. |